Rafael Santana Nunes, Diogo Vila-Viçosa, and Paulo J. Costa
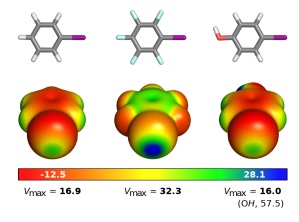
Halogen bonds (HaBs) are noncovalent interactions where halogen atoms act as electrophilic species interacting with Lewis bases. These interactions are relevant in biochemical systems being increasingly explored in drug discovery, mainly to modulate protein–ligand interactions. In this work, we report evidence for the existence of HaB-mediated halogen–phospholipid recognition phenomena as our molecular dynamics simulations support the existence of favorable interactions between halobenzene derivatives and both phosphate (PO) or ester (CO) oxygen acceptors from model phospholipid bilayers, thus providing insights into the role of HaBs in driving the permeation of halogenated drug-like molecules across biological membranes. This represents a relevant molecular mechanism, previously overlooked, determining the pharmacological activity of halogenated molecules with implications in drug discovery and development, a place where halogenated molecules account for a significant part of the chemical space. Our data also shows that, as the ubiquitous hydrogen bond, HaBs should be accounted for in the development of membrane permeability models.
Doi: 10.26434/chemrxiv.11894547.v1
Cited as: Nunes R, Vila Viçosa D, Costa PJ (2020) Halogen Bonding: An Underestimated Player in Membrane–drug Interactions. ChemRxiv. Preprint; https://doi.org/10.26434/chemrxiv.11894547.v1.