Jessica C. McGlynn, Torben Dankwort, Lorenz Kienle, Nuno A. G. Bandeira, James P. Fraser, Emma K. Gibson, Irene Cascallana-Matías, Katalin Kamarás, Mark D. Symes, Harry N. Miras & Alexey Y. Ganin
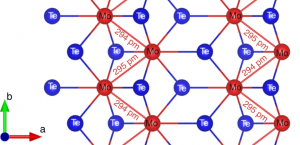
The electrochemical generation of hydrogen is a key enabling technology for the production of sustainable fuels. Transition metal chalcogenides show considerable promise as catalysts for this reaction, but to date there are very few reports of tellurides in this context, and none of these transition metal telluride catalysts are especially active. Here, we show that the catalytic performance of metallic 1T′-MoTe2 is improved dramatically when the electrode is held at cathodic bias. As a result, the overpotential required to maintain a current density of 10 mA cm−2 decreases from 320 mV to just 178 mV. We show that this rapid and reversible activation process has its origins in adsorption of H onto Te sites on the surface of 1T′-MoTe2. This activation process highlights the importance of subtle changes in the electronic structure of an electrode material and how these can influence the subsequent electrocatalytic activity that is displayed.