Oliveira S, Bandeira NAG, Leal JP, Maria L, Carretas JM, Monteiro B, Marçalo J.
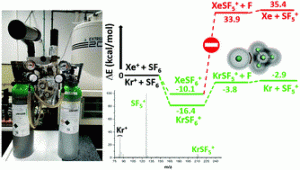
The gas-phase reactions of noble gas (Ng) cations, namely Kr+ and Xe+, with SF6 were investigated experimentally by Fourier transform ion cyclotron resonance mass spectrometry and computationally using RI-MP2 and BCCD(T) methods. The study revealed a new interaction between Kr+ and neutral SF6 that gave rise to a new cationic, weakly bound complex of Kr, [KrSF5]+, although the major reaction channel was dissociative electron transfer to yield SF5+ and {Kr, F}. Experimental studies examined the formation and stability of the new species and computational studies addressed the energetics of the reaction and indicated that [KrSF5]+ is stable by ca. 1 kcal mol−1. The same computational approach was used to examine the reaction of Xe+ with SF6 and showed it to be thermodynamically unfavourable by ca. 35 kcal mol−1, confirming the non-observation of reaction in the mass spectrometry experiments. An analysis of the bonding in [KrSF5]+ clearly showed that it is a non-covalently bound species, while in its presumed precursor [KrSF6]+ a partially covalent Kr–F bond is present.
Doi: 10.1039/d1cp05814b
Oliveira S, Bandeira NAG, Leal JP, Maria L, Carretas JM, Monteiro B, Marçalo M (2022) A new krypton complex – experimental and computational investigation of the krypton sulphur pentafluoride cation, [KrSF5]+, in the gas phase. Physical Chemistry Chemical Physics, , . doi: 10.1039/d1cp05814b