World Cancer Day | February 4
[text by Paulo Matos, Principal Investigator from Gene Expression & Regulation Research Group at BioISI ]
World Cancer Day: towards a more equitable society
Cancer refers to any one of a large number of diseases characterized by the development of abnormal cells that divide uncontrollably, acquiring the ability to infiltrate and destroy normal body tissue. Cancer is the second-leading cause of death in the world and the World Cancer Day initiative, held on February 4th, is led by the Union for International Cancer Control (UICC) to raise worldwide awareness and mobilize society to reimagine a world where millions of preventable cancer deaths are saved and access to life-saving cancer treatment and care is equitable for all – no matter who you are or where you live.
Colorectal Cancer Research at Peter Jordan’s Lab (National Institute of Health Dr. Ricardo Jorge)
According to the latest estimates, colorectal cancer has become the neoplasia with the highest incidence in Portugal. Most often, colorectal cancer originates from polyps – small clumps of cells that form on the lining of the colon. Anyone can develop colon polyps, particularly when over 50 years old, but most colon polyps are harmless. However, over time, some of these polyps can develop into colon cancer. At BioISI, the team of Peter Jordan, principal investigator from the Department of Human Genetics (R&D Unit), at the National Institute of Health Dr. Ricardo Jorge (INSA), has been studying colorectal cancer for over 20 years. We’ve screened hundreds of tumors to identify mutations in critical genes known to regulate normal cell behavior. Some of these genes are called oncogenes because when altered they become hyperactive, stimulating abnormal cellular multiplication and survival.

Peter Jordan’s Lab Team [images provided by Paulo Matos]
Can cancer cells become dormant?
Another important group of genes are called tumor suppressors. Like the name says, these genes act to suppress abnormal cell division driven, for instance, by mutated oncogenes. They often trigger a defense program known as “oncogene-induced senescence” (OIS), which means that they make cells bearing mutations in certain oncogenes become “dormant” (senescent), thus suppressing abnormal growth. That is why, besides activating mutations in oncogenes, most cancers also carry inactivating mutations in tumor suppressor genes.
Alternative splicing: when genes with the same letters “write” different words
About 15 years ago, while searching for mutations in a new potential oncogene called RAC1, the team found something unexpected: although no mutations where detected, in several tumors, the message written in that potential oncogene was, nonetheless, being altered!
Further investigation revealed that this occurred through a mechanism called “alternative splicing”. What is it? Well, the messages encoded in genes in our DNA are split in several pieces that have to be “spliced” together in the proper order to make the correct “word”. Take for instance the word “PLAYER”; move two letters and it becomes “REPLAY” – an “alternative” letter “splicing” led to a similar word with a different meaning. A similar thing happened to RAC1’s message in those tumors – it was alternatively spliced into a slightly different variant that the team called RAC1B.
The serrated colorectal cancer pathway
Further research led us to discover that the RAC1B variant was not only hyperactive but also highly focused on cell multiplication and survival signals. When we experimentally removed it from colorectal cancer cells these stopped dividing and quickly died. Moreover, when we artificially introduced RAC1B into lab cultures of normal cells it increased the rate of cell division and allowed cells to survive even when starved for nutrients. After studying a large number of colorectal tumor samples, our team observed that RAC1B appeared most frequently in tumors that carried an activating mutation in a particular oncogene called BRAF. Tumors bearing this oncogene mutation appear more frequently on the proximal colon (i.e., right side) and usually arise from neoplastic polyps that have a distinct saw-tooth-like appearance under the microscope – termed serrated polyps. Cells carrying the BRAF mutation tend to become senescent due to the activation of the above-mentioned OIS-inducing cellular mechanism. Because most of the BRAF tumors (in which tumor cells had to overcome OIS to multiply) also had increased RAC1B levels, the team reasoned that perhaps RAC1B appearance was the wakeup call that took these cells from their dormant state! We tested this in normal colon cells where we introduced either mutant BRAF alone or together with RAC1B. Cells with only mutant BRAF became senescent after only a few divisions but those where RAC1B was also present continued to multiply happily! And here was the proof that RAC1B was the culprit for taking BRAF mutant colon cells out of “hibernation”!
If this part was a bit confusing, please see the figure below depicting the serrated colorectal cancer pathway.
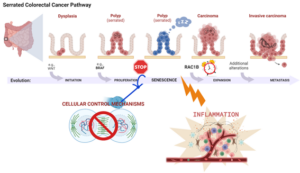
Figure [provided by Paulo Matos] – the serrated colorectal cancer pathway| Molecular changes in colon mucosa cells alter their functioning and morphology (dysplasia). In the ascending colon (right side) some of these changes are accompanied by activation mutations in genes, like BRAF, that promote further dysregulated cell division leading to the formation of polyps, some of which showing a saw-tooth-like appearance under the microscope (serrated polyps). This abnormal cell division triggers control mechanisms inside the cell that stop cells from dividing and render them senescent (dormant). But stimuli coming from the surrounding microenvironment can overcome this dormancy. One of these is inflammation. The inflammatory signals induce several changes in the dormant cells, including the production of the RAC1B variant. This variant obviates the cell division block and brings cells out of senescence. It reignites dysregulated multiplication and enables tumor cells to survive even in harsh conditions. This allows tumor cells to gain additional genetic changes that, ultimately, lead to malignant progression and metastasis.
Can a well-known anti-inflammatory drug help?
But how was RAC1B being triggered in the colon? What changed for it to suddenly appear? The first clue came when the team discovered high levels of RAC1B in colon biopsies from patients with inflammatory bowel disease. Moreover, when we experimentally induced acute colitis in mice, the inflamed colon tissue also presented increased RAC1B levels. We then went back to lab cultures and exposed colon cells to activated inflammatory cells and, as expected, RAC1B readily popped up! The team then thoroughly investigated what signals were being exchanged between colon and inflammatory cells and identified interleukin 6 (IL-6; a pro-inflammatory molecule secreted by immune cells) as the signal that made RAC1B increase in colorectal tumor cells. Chronic inflammation has been associated with increased risk for several types of cancer, including colorectal cancer. Some studies have reported that certain anti-inflammatory drugs, many of which reduce IL-6 levels, can, in some cases, help reduce the risk of colorectal cancer. By investigating this aspect our team found that the very well-known anti-inflammatory drug ibuprofen can not only reduce IL-6 levels but can also enter colon cells and directly interfere with the mechanism that generates the RAC1B splice variant.
The next steps in BioISI cancer research
In summary, the BioISI team’s research has allowed the characterization of new molecular mechanisms behind serrated colorectal cancer and identified a known drug that can be used to improve treatment for some of these patients. The team now continues to study how inflammation promotes colorectal cancer development, looking for new ways to fight this increasingly prevalent disease.
Find out more about the World Cancer Day 2023 edition here.