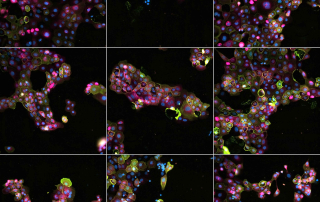
The Facility provides an integrated environment for research projects which interrogate large compound or nucleic acid libraries or require agile or robotized sample handling. Facility staff provides support at all stages of a screening campaign from assay design to sample preparation, instrument operation, bioimage quantification, data analysis and software development. The Facility also offers advanced training events on microscopy, high content screening and bioimage analysis.
SAMPLE PREPARATION
A set of sample preparation technologies enable efficient handling of cell-based assays and library management. Once data is acquired, short-term local storage is offered for screening datasets.

HIGH-CONTENT AND HIGH-THROUGHPUT SCREENING
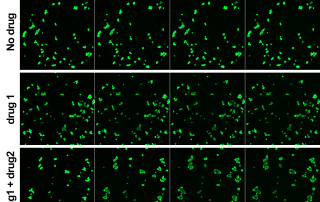
The Facility hosts screening projects based on microscopy or plate reader readouts. Image-based assays provide rich phenotypic data for individual cells (high content). Our imaging systems are capable of live cell assays and multimodal imaging. High-throughput plate reader measurements (absorption, fluorescence, luminescence) enable efficient screening projects which can be combined with fast online reagent injection.
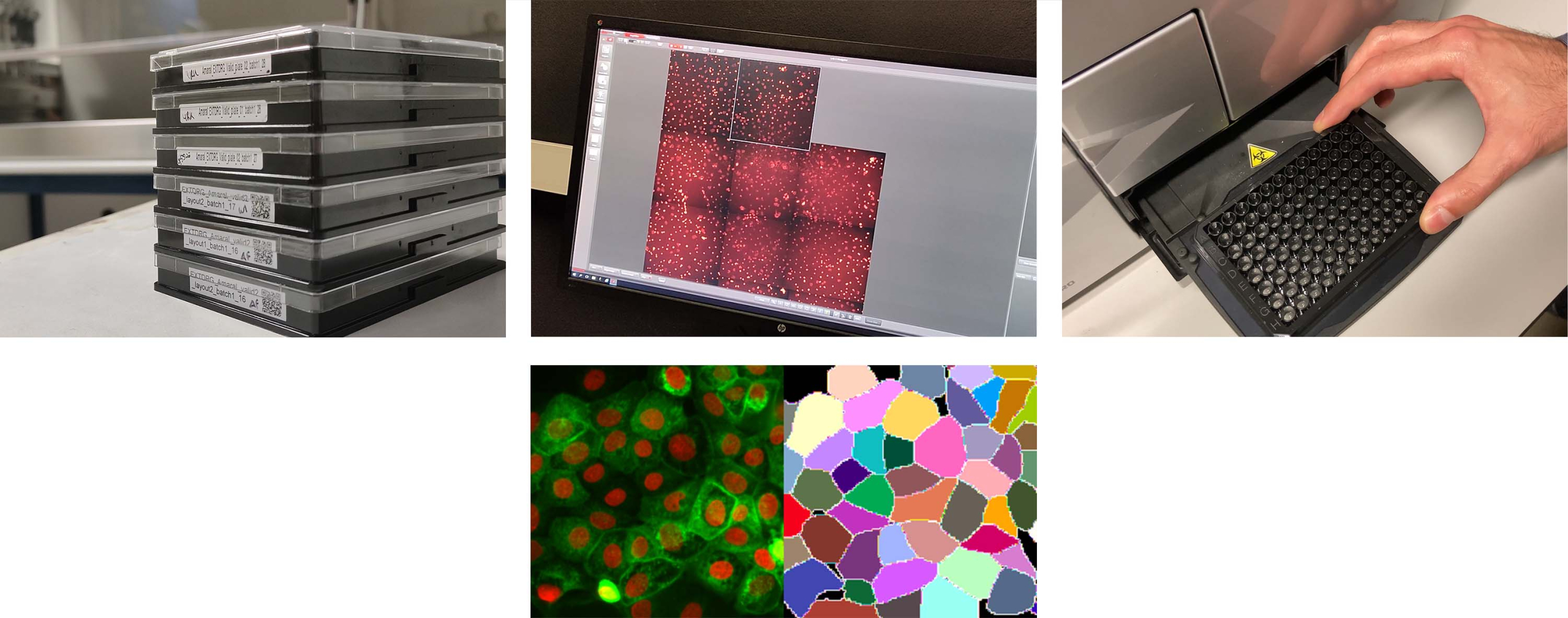
BIOIMAGING
Microscopy systems provide imaging capabilities for screening or non-screening projects. Most systems are fully motorized and compatible with live specimens.

Available imaging modalities:
- Brightfield Microscopy (phase contrast, DIC)
- Widefield fluorescence
- Confocal fluorescence
- Stereomicroscopy
- Fluorescence Lifetime Imaging (FLIM)
- Förster Resonance Energy Transfer imaging (FRET)
- High-throughput microscopy
- Live cell imaging
BIOIMAGE ANALYSIS
The Facility provides support on bioimage analysis, with a focus on large imaging datasets. Our users have the oportunity to be trained on the tools which better solve their biological requirements. We have a track record on the development of assay-specific bioimage analysis pipelines, exploratory data analysis software and statistical data analysis.
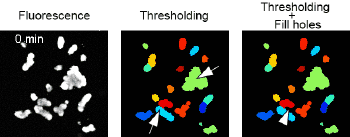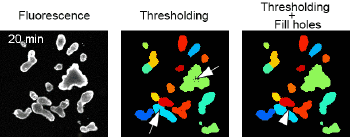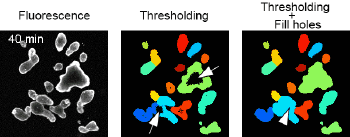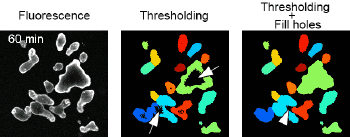
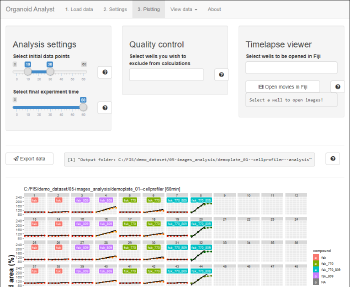
APPLICATIONS
- Drug screening
- Natural product screening
- Functional genomics
- siRNA screening
- Cytotoxicity/viability determinations
- Cell phenotyping
- Live cell imaging
- Automated microscopy experiments
NATIONAL AND INTERNATIONAL CONSORTIA
The High-Throughput Screening Facility integrates the National Roadmap of Research Infrastructures (PPBI and PT-OPENSCREEN), as well as the European Research Infrastructure Consortium (Euro-BioImaging ERIC and EU-OPENSCREEN ERIC).
Links
- Bacalhau M, Ferreira FC, Kmit, A, Souza FR, da Silva VD, Pimentel AS, Amaral MD, Buarque CD, Lopes-Pacheco M (2023) Identification of novel F508del-CFTR traffic correctors among triazole derivatives. Eur J Pharmacol, 938:175396. DOI: 10.1016/j.ejphar.2022.175396
- Rodenburg LW, Delpiano L, Railean V, Centeio R, Pinto MC, Smits SMA, van der Windt IS, van Hugten CFJ, van Beuningen SFB, Rodenburg RNP, van der Ent CK, Amaral MD, Kunzelmann K, Gray MA, Beekman JM, Amatngalim GD (2022) Drug Repurposing for Cystic Fibrosis: Identification of Drugs That Induce CFTR-Independent Fluid Secretion in Nasal Organoids. Int J Mol Sci, 23(20):12657. DOI: 10.3390/ijms232012657
- Godinho-Pereira J, Lopes MD, Garcia AR, Botelho HM, Malhó R, Figueira I, Brito MA (2022) A Drug Screening Reveals Minocycline Hydrochloride as a Therapeutic Option to Prevent Breast Cancer Cells Extravasation across the Blood–Brain Barrier. Biomedicines, 10(8):1988. DOI: 10.3390/biomedicines10081988
- Ferreira JF, Silva IAL, Botelho HM, Amaral MD, Farinha CM (2022) Absence of EPAC1 Signaling to Stabilize CFTR in Intestinal Organoids. Cells, 11(15):2295. DOI: 10.3390/cells11152295
- Fernandes C, Palma E, Silva F, Belchior A, Pinto CIG, Guerreiro FJ, Botelho HM, Mendes F, Raposinho P, Paulo A (2022) Searching for a Paradigm Shift in Auger-Electron Cancer Therapy with Tumor-Specific Radiopeptides Targeting the Mitochondria and/or the Cell Nucleus. Int J Mol Sci, 23(13):7238. DOI: 10.3390/ijms23137238
- Quaresma MC, Botelho HM, Pankonien I, Rodrigues CS, Pinto MC, Costa PR, Duarte A, Amaral MD (2022) Exploring YAP1-centered networks linking dysfunctional CFTR to epithelial–mesenchymal transition. Life Sci Alliance, 5(9):e202101326. DOI: 10.26508/lsa.202101326
- Lim SH, Snider J, Birimberg-Schwartz L, Ip W, Serralha JC, Botelho HM, Lopes-Pacheco M, Pinto MC, Moutaoufik MT, Zilocchi M, Laselva O, Esmaeili M, Kotlyar M, Lyakisheva A, Tang P, Vázquez LL, Akula I, Aboualizadeh F, Wong V, Grozavu I, Opacak-Bernardi T, Yao Z, Mendoza M, Babu M, Jurisica I, Gonska T, Bear CE, Amaral MD, Stagljar I (2022) CFTR interactome mapping using the mammalian membrane two-hybrid high-throughput screening system. Mol Syst Biol, 18(2):e10629. DOI: 10.15252/msb.202110629
- Pinto MC, Botelho HM, Silva IAL, Railean V, Neumann B, Pepperkok R, Schreiber R, Kunzelmann K, Amaral MD (2022) Systems Approaches to Unravel Molecular Function: High-content siRNA Screen Identifies TMEM16A Traffic Regulators as Potential Drug Targets for Cystic Fibrosis. J Mol Biol, 434(5):167436. DOI: 10.1016/j.jmb.2021.167436
- Lopes-Pacheco M, Bacalhau M, Ramalho SS, Silva IAL, Ferreira FC, Carlile GW, Thomas DY, Farinha CM, Hanrahan JW, Amaral MD (2022) Rescue of Mutant CFTR Trafficking Defect by the Investigational Compound MCG1516A. Cells, 11(1):136. DOI: 10.3390/cells11010136
- Ramalho SS, Silva IAL, Amaral MD, Farinha CM (2022) Rare Trafficking CFTR Mutations Involve Distinct Cellular Retention Machineries and Require Different Rescuing Strategies. Int J Mol Sci, 23(1):24. DOI: 10.3390/ijms23010024
- Simões FB, Kmit A, Amaral MD (2021) Cross-talk of inflammatory mediators and airway epithelium reveals the cystic fibrosis transmembrane conductance regulator as a major target. ERJ Open Res, 7(4):00247-2021. DOI: 10.1183/23120541.00247-2021
- Pinto MC, Quaresma MC, Silva IAL, Railean V, Ramalho SS, Amaral MD (2021) Synergy in Cystic Fibrosis Therapies: Targeting SLC26A9. Int J Mol Sci, 22(33):13064. DOI: 10.3390/ijms222313064
- Moreira GG, Cantrelle F-X, Quezada A, Carvalho FS, Cristóvão JS, Sengupta U, Puangmalai N, Carapeto AP, Rodrigues MS, Cardoso I, Fritz G, Herrera F, Kayed R, Landrieu I, Gomes CM (2021) Dynamic interactions and Ca2+-binding modulate the holdase-type chaperone activity of S100B preventing tau aggregation and seeding. Nat Commun, 12(1):6292. DOI: 10.1038/s41467-021-26584-2
- Santos L, Mention K, Cavusoglu-Doran K, Sanz DJ, Bacalhau M, Lopes-Pacheco M, Harrison PT, Farinha CM (2021) Comparison of Cas9 and Cas12a CRISPR editing methods to correct the W1282X-CFTR mutation. J Cyst Fibros, in press:. DOI: 10.1016/j.jcf.2021.05.014
- Silva IAL, Railean V, Duarte A, Amaral MD (2021) Personalized Medicine Based on Nasal Epithelial Cells: Comparative Studies with Rectal Biopsies and Intestinal Organoids. J Pers Med, 11(5):421. DOI: 10.3390/jpm11050421
- Serrazina S, Machado H, Costa RL, Duque P, Malhó R (2021) Expression of Castanea crenata allene oxide synthase in Arabidopsis improves the defense to Phytophthora cinnamomi. Front Plant Sci, 12:628697. DOI: 10.3389/fpls.2021.628697
- Hagemeijer MC, Vonk AM, Awatade NT, Silva IAL, Tischer C, Hilsenstein V, Beekman JM, Amaral MD, Botelho HM (2020) An open-source high-content analysis workflow for CFTR function measurements using the forskolin-induced swelling assay. Bioinformatics, :btaa1073. DOI: 10.1093/bioinformatics/btaa1073
- Silva IAL, Duarte A, Marson FAL, Centeio R, Doušová T, Kunzelmann K, Amaral MD (2020) Assessment of distinct electrophysiological parameters in rectal biopsies for the choice of the best diagnosis/prognosis biomarkers for cystic fibrosis. Front Physiol, 11:604580. DOI: 10.3389/fphys.2020.604580
- Quaresma MC, Pankonien I, Clarke LA, Sousa LS, Silva IAL, Railean V, Doušová T, Fuxe J, Amaral MD (2020) Mutant CFTR drives TWIST1 mediated epithelial-mesenchymal transition. Cell Death Dis, 11(10):920. DOI: 10.1038/s41419-020-03119-z
- Sousa L, Pankonien I, Simões FB, Chanson M, Amaral MD (2020) Impact of KLF4 on Cell Proliferation and Epithelial Differentiation in the Context of Cystic Fibrosis. Int J Mol Sci, 21(18):6717. DOI: 10.3390/ijms21186717
- Silva IAL, Doušová T, Ramalho S, Centeio R, Clarke LA, Railean V, Botelho HM, Holubová A, Valášková I, Yeh J-T, Hwang T-C, Farinha CM, Kunzelmann K, Amaral MD (2020) Organoids as a personalized medicine tool for ultra-rare mutations in cystic fibrosis: The case of S955P and 1717-2A>G. Biochim Biophys Acta Mol Basis Dis, 1866(11):165905. DOI: 10.1016/j.bbadis.2020.165905
- Santos JD, Pinto FR, Ferreira JF, Amaral MD, Zaccolo M, Farinha CM (2020) Cytoskeleton regulators CAPZA2 and INF2 associate with CFTR to control its plasma membrane levels under EPAC1 activation. Biochem J, 477(13):2561–2580. DOI: 10.1042/BCJ20200287
- Lopes-Pacheco M, Silva IAL, Turner MJ, Carlile GW, Sondo E, Thomas DY, Pedemonte N, Hanrahan JW, Amaral MD (2020) Characterization of the mechanism of action of RDR01752, a novel corrector of F508del-CFTR. Biochem Pharmacol, 180:114133. DOI: 10.1016/j.bcp.2020.114133
- Sousa L, Pankonien I, Clarke LA, Silva I, Kunzelmann K, Amaral MD (2020) KLF4 acts as a wt-CFTR suppressor through an AKT-mediated pathway. Cells, 9(7):1607. DOI: 10.3390/cells9071607
- Vonk AM, van Mourik P, Ramalho AS, Silva IAL, Statia M, Kruisselbrink E, Suen SWF, Dekkers JF, Vleggaar FP, Houwen RHJ, Mullenders J, Boj SF, Vries R, Amaral MD, de Boeck K, van der Ent CK, Beekman JM (2020) Protocol for application, standardization and validation of the forskolin-induced swelling assay in cystic fibrosis human colon organoids. STAR Protoc, 1(1):100019. DOI: 10.1016/j.xpro.2020.100019
- Figueiredo AM, Villacampa P, Diéguez-Hurtado R, Lozano JJ, Kobialka P, Cortazar AR, Martinez-Romero A, Angulo-Urarte A, Franco CA, Claret M, Aransay AM, Adams RH, Carracedo A, Graupera M (2020) Phosphoinositide 3-Kinase–Regulated Pericyte Maturation Governs Vascular Remodeling. Circulation, 142:688–704. DOI: 10.1161/CIRCULATIONAHA.119.042354
- Pinto MC, Schreiber R, Lérias J, Ousingsawat J, Duarte A, Amaral M, Kunzelmann K (2020) Regulation of TMEM16A by CK2 and its role in cellular proliferation. Cells, 9(5):1138. DOI: 10.3390/cells9051138
- Correia S, Santos M, Glińska S, Gapińska M, Matos M, Carnide V, Schouten R, Silva AP, Gonçalves B (2020) Effects of exogenous compound sprays on cherry cracking: skin properties and gene expression. J Sci Food Agric, 100(7):2911–2921. DOI: 10.1002/jsfa.10318
- Simões FB, Quaresma MC, Clarke LA, Silva IAL, Pankonien I, Railean V, Kmit A, Amaral MD (2019) TMEM16A chloride channel does not drive mucus production. Life Sci Alliance, 2(6):e201900462 1. DOI: 10.26508/lsa.201900462
- Amaral MD, Hutt DM, Tomati V, Botelho HM, Pedemonte N (2020) CFTR processing, trafficking and interactions. J Cyst Fibros, 19 Suppl 1:S33–S36. DOI: 10.1016/j.jcf.2019.10.017
- Amaral MD, Beekman JM (2020) Activating alternative chloride channels to treat CF: Friends or Foes?: Report on the Meeting of the Basic Science Working Group in Dubrovnik, Croatia. J Cyst Fibros, 19(1):11–15. DOI: 10.1016/j.jcf.2019.10.005
- Garcia AR, Godinho-Pereira J, Figueira I, Malhó R, Brito MA (2019) Replicating the blood- brain barrier properties in an in vitro model: effects of hydrocortisone and/or shear stress. Archives of Anatomy, 8(2):1-20.
- Loureiro CA, Santos JD, Matos AM, Jordan P, Matos P, Farinha CM, Pinto FR (2019) Network biology identifies novel regulators of CFTR trafficking and membrane stability. Front Pharmacol, 10:619. DOI: 10.3389/fphar.2019.00619
- Santos JD, Canato S, Carvalho AS, Botelho HM, Aloria K, Amaral MD, Matthiesen R, Falcão AO, Farinha CM (2019) Folding status is determinant over traffic-competence in defining CFTR interactors in the endoplasmic reticulum. Cells, 8(4):353. DOI: 10.3390/cells8040353
- Patel W, Moore PJ, Sassano MF, Lopes-Pacheco M, Aleksandrov AA, Amaral MD, Tarran R, Gray MA (2019) Increases in cytosolic Ca2+ induce dynamin- and calcineurin-dependent internalisation of CFTR. Cell Mol Life Sci, 76(5):977–994. DOI: 10.1007/s00018-018-2989-3
- Palma E, Botelho HM, Morais GR, Rodrigues I, Santos IC, Campello MPC, Raposinho P, Belchior A, Gomes SS, Araújo MF, Correia I, Ribeiro N, Gama S, Mendes F, Paulo A (2019) Unravelling the antitumoral potential of novel bis(thiosemicarbazonato) Zn(II) complexes: structural and cellular studies. J Biol Inorg Chem, 24(1):71–89. DOI: 10.1007/s00775-018-1629-6
- Awatade NT, Ramalho S, Silva IAL, Felício V, Botelho HM, de Poel E, Vonk A, Beekman JM, Farinha CM, Amaral MD (2019) R560S: A class II CFTR mutation that is not rescued by current modulators. J Cyst Fibros, 18(2):182–189. DOI: 10.1016/j.jcf.2018.07.001
- Olivença DV, Uliyakina I, Fonseca LL, Amaral MD, Voit EO, Pinto FR (2018) A mathematical model of the phosphoinositide pathway. Sci Rep, 8(1):3904. DOI: 10.1038/s41598-018-22226-8
- Lérias JR, Pinto MC, Botelho HM, Awatade NT, Quaresma MC, Silva IAL, Wanitchakool P, Schreiber R, Pepperkok R, Kunzelmann K, Amaral MD (2018) A novel microscopy-based assay identifies extended synaptotagmin-1 (ESYT1) as a positive regulator of anoctamin 1 traffic. Biochim Biophys Acta Mol Cell Res, 1865(2):421–431. DOI: 10.1016/j.bbamcr.2017.11.009
- Amaral MD, Farinha CM, Matos P, Botelho HM (2016) Investigating alternative transport of integral plasma membrane proteins from the ER to the Golgi: lessons from the cystic fibrosis transmembrane conductance regulator (CFTR). Methods Mol Biol, 1459:105–126. DOI: 10.1007/978-1-4939-3804-9_7
- Silva AC, Rodrigues SC, Caldeira J, Nunes AM, Sampaio-Pinto V, Resende TP, Oliveira MJ, Barbosa MA, Thorsteindóttir S, Pinto-do-Ó P (2016) Three-dimensional scaffolds of fetal decellularized hearts exhibit enhanced potential to support cardiac cells in comparison to the adult. Biomaterials, 104:52–64. DOI: 10.1016/j.biomaterials.2016.06.062
- Igreja S, Clarke LA, Botelho HM, Marques L, Amaral MD (2016) Correction of a cystic fibrosis splicing mutation by antisense oligonucleotides. Human Mutat, 37(2):209–215. DOI: 10.1002/humu.22931
- Amaral MD, Balch WE (2015) Hallmarks of therapeutic management of the cystic fibrosis functional landscape. J Cyst Fibros, 14(6):687–699. DOI: 10.1016/j.jcf.2015.09.006
- Verkman AS, Edelman A, Amaral M, Mall MA, Beekman JM, Meiners T, Galietta LJV, Bear CE (2015) Finding new drugs to enhance anion secretion in cystic fibrosis: Toward suitable systems for better drug screening. Report on the pre-conference meeting to the 12th ECFS Basic Science Conference, Albufeira, 25-28 March 2015. J Cyst Fibros, 14(6):700–705. DOI: 10.1016/j.jcf.2015.10.001
- Clarke LA, Botelho HM, Sousa L, Falcão AO, Amaral MD (2015) Transcriptome meta-analysis reveals common differential and global gene expression profiles in cystic fibrosis and other respiratory disorders and identifies CFTR regulators. Genomics, 106(5):268–277. DOI: 10.1016/j.ygeno.2015.07.005
- Botelho HM, Uliyakina I, Awatade NT, Proença MC, Tischer C, Sirianant L, Kunzelmann K, Pepperkok R, Amaral MD (2015) Protein traffic disorders: an effective high-throughput fluorescence microscopy pipeline for drug discovery. Sci Rep, 5(1):9038. DOI: 10.1038/srep09038