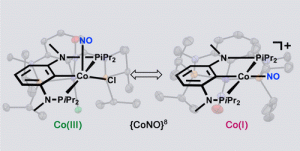
Jan Pecak, Wolfgang Eder, Berthold Stöger, Sara Realista, Paulo N. Martinho, Maria José Calhorda, Wolfgang Linert, and Karl Kirchner
Doi: 10.1021/acs.organomet.0c00167
Cited as: Pecak J, Eder W, Stöger B, Realista S, Martinho PN, Calhorda MJ, Linert W, Kirchner K (2020) Synthesis, Characterization, and Catalytic Reactivity of {CoNO}8 PCP Pincer Complexes. Organometallics 39, 14, 2594–2601; https://doi.org/10.1021/acs.organomet.0c00167.